We have over 25 years of experience.
By WHO
Category: Health, Industry
 No Comments
No Comments
New US$50 billion health, trade and finance roadmap to end the pandemic and secure a global recovery
Heads of International Monetary Fund (IMT), World Bank Group (WB), World Health Organization (WHO) and World Trade Organization (WTO) issue extraordinary call for financing actions by government leaders to accelerate end to COVID-19 pandemic.
IMF, WB, WHO and WTO principals call for US$50 billion investment to generate US$9 trillion in global economic returns by 2025 and boost manufacturing capacity, supply, trade flows and the equitable distribution of diagnostics, oxygen, treatments, medical supplies and vaccines.
Call to action by this quadrilateral grouping comes at a perilous point in the pandemic and as the historic World Health Assembly concludes, G7 meetings commence, and follows the G20 Global Health Summit.
Doses need to be donated immediately to developing countries, synchronized with national vaccine deployment plans, including through COVAX, which is co-led by CEPI, Gavi and WHO, alongside key delivery partner UNICEF.
The heads of the world’s predominant global financing, health and trade agencies have united to urge government leaders to urgently finance a new US$50 billion roadmap to accelerate the equitable distribution of health tools to help end the pandemic that has devastated lives and livelihoods for 18 months and also set the foundations for a truly global recovery, as well as enhanced health security.
In a statement published by newspapers around the world, the leaders of the International Monetary Fund, World Bank Group, World Health Organization and World Trade Organization [Kristalina Georgieva, Tedros Adhanom Ghebreyesus, David Malpass and Ngozi Okonjo-Iweala] said governments must act without further delay or risk continued waves and explosive outbreaks of COVID-19 as well as more transmissible and deadly virus variants undermining the global recovery.
Leaders of the four agencies said: “By now it has become abundantly clear there will be no broad-based recovery without an end to the health crisis. Access to vaccination is key to both.”
The joint statement draws on a recent IMF staff analysis, which stated that US$50 billion in new investment is needed to increase manufacturing capacity, supply, trade flows, and delivery, which would accelerate the equitable distribution of diagnostics, oxygen, treatments, medical supplies and vaccines. This injection would also give a major boost to economic growth around the world.
“At an estimated US$50 billion, it will bring the pandemic to an end faster in the developing world, reduce infections and loss of lives, accelerate the economic recovery, and generate some US$9 trillion in additional global output by 2025, “said the leadership."
It echoes economic analysis by the International Chamber of Commerce; and the Eurasia Group – both of which make the case for a relatively modest investment by governments in comparison to the trillions spent on national stimulus plans and lost trillions in foregone economic output. But the critical element of this is that it effectively spurs global vaccination and bridges the equity gap.
“Increasing our ambition and vaccinating more people faster: WHO and its COVAX partners have set a goal of vaccinating approximately 30% of the population in all countries by the end of 2021,” said the four leaders. “But this can reach even 40% through other agreements and surge investment, and at least 60 percent by the first half of 2022.”
Governments are urged to act on the investment opportunity to boost supplies of vaccines, oxygen, tests and treatment. The IMF, WBG, WHO and WTO leaders issued their joint statement as the World Health Assembly drew to a conclusion and a round of G7 meetings were set to start, beginning with a Finance Ministers convening later this week, and following a Global Health Summit co-hosted by the EU and Italy, which chairs the G20.
“To urgently get more shots in arms, doses need to be donated immediately to developing countries synchronized with national vaccine deployment plans, including through COVAX,” said the four leaders. “Cooperation on trade is also needed to ensure free cross-border flows and increasing supplies of raw materials and finished vaccines” roadmap to ending the pandemic and driving a truly global and fast recovery.
By Xinhua
Category: Business, Industry
 No Comments
No Comments
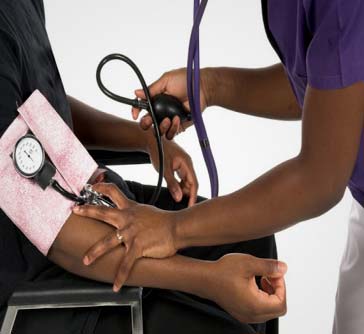
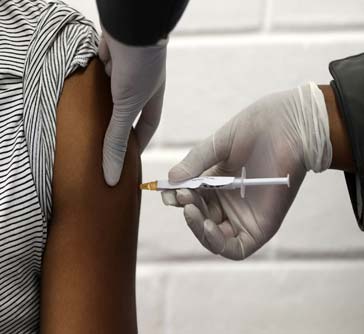
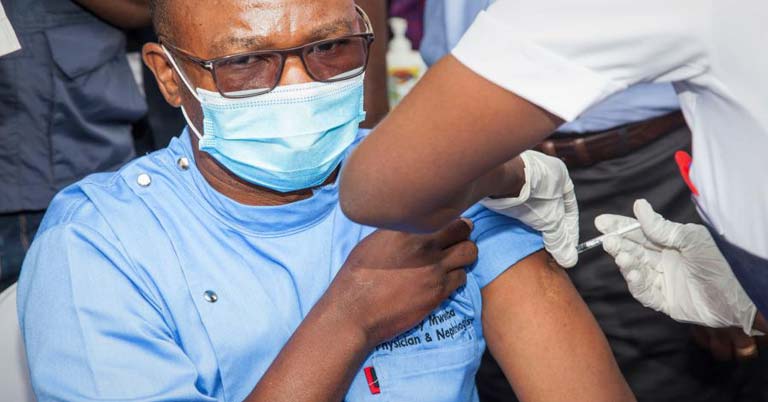
Zambia on Wednesday launched the COVID-19 vaccination program against the pandemic.
The national launch, which was held at the University Teaching Hospital (UTH), the country's highest referral hospital in the country's capital Lusaka, was attended by officials from the health ministry, cooperating partners and private sector representatives.
The launch involves the first pillar of the program under the COVAX Facility consisting of 228,000 doses of AstraZeneca manufactured in India.
The first pillar will cover 20 percent or 3.6 million of the eligible over 18 years people and will target front-line health workers and other people at most risk. A total of 8.3 million people are targeted to be vaccinated.

By Zambia Daily Mail
Category: Business, Industry
 No Comments
No Comments
AFRICA, now more than before, needs robust health care systems to keep its populations healthy and productive in the face of COVID-19.President Edgar Lungu is on point in calling for African leaders to focus on building their respective countries’ health care capacities by training more health workers in various specialities to keep the continent’s population productive.As the President has noted, with Africa’s population growing to almost 1.4 billion, leaders on the continent should prioritise strengthening their health care systems.
He urges “African leaders and all of you to focus on building our health care capacity and train more and more health care providers in various specialties to keep the African population healthy and productive.”Needless to say, Africa needs a healthy and productive population to push its development agenda.And at a time that Africa is battling with the COVID-19 pandemic, the continent cannot afford to lag behind in terms of health care capacity. While in the past it was a matter of one having resources and they could access specialised health care services from across the globe, COVID-19 has dawned a new reality that Africa can no longer depend on the Western world for specialised health care services as much as it did before.
During times of COVID, the overwhelming demand for health care services, coupled with restrictions on travels, has made it difficult for Africans, even the wealthy, to trot around the globe in pursuit of health care services. It is a wake-up call for African countries to build health care capacity and much more that its population is growing exponentially. Many African countries are grappling with inadequate health manpower and infrastructure. While there has been some progress over the years, many countries in Africa still have a shortage of specialised health workers in various areas.
Some countries still do not have capacity to conduct specialised and delicate surgeries. Zambia has been making steady progress in this area, with a growing number of delicate surgeries successfully conducted at the University Teaching Hospitals. There’s need to build more capacity in such areas. The continent needs to rise to the challenge and develop world-class health care systems which can holistically meet the health needs of its populations.
This calls for more investment in health infrastructure development and specialised training for health personnel. There is also need for African countries to allocate more resources to drugs and other medical supplies.
The continent needs to set targets for itself on building its health care capacity and work towards achieving them in a specified period. As long as the continent cannot sufficiently handle its health needs, development will be far-fetched. The continent needs a healthy and productive population that can push its development agenda.
Africa, despite being lavishly endowed with natural resources, has struggled to fully exploit them to spur its development. One of the many reasons is the disease burden which keeps claiming the productive age. COVID-19 has only added to the health burden, depriving families of breadwinners. Organisations and nations have been deprived of productive members, too.
“I believe that stronger political commitment, purposeful and strategic partnerships are key to improving health outcomes and the well-being of all people in the world, especially in Africa,” President Lungu said. The head of State is on point because this is not an agenda that can be driven in isolation by individual countries. There’s need for partnerships and solidarity, especially that all countries are working towards achieving the same goals in line with the United Nations Agenda 2030 on Sustainable Development.
African countries must all rise to the challenge and put a premium on the health agenda for a more healthy and productive population.
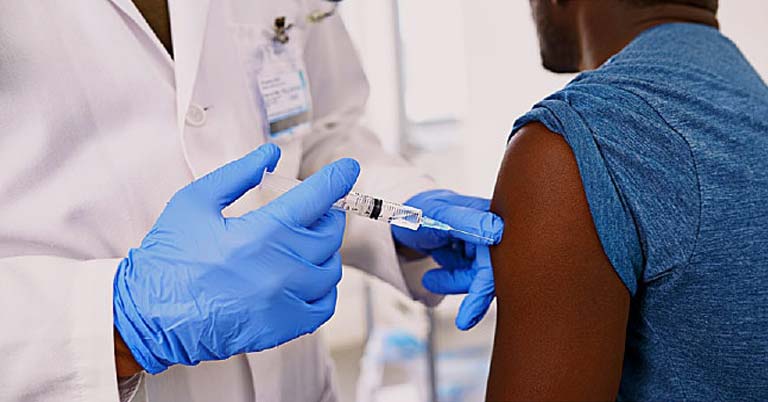
By WHO
Category: Health, Industry
 No Comments
No Comments
WHO today validated the Sinovac-CoronaVac COVID-19 vaccine for emergency use, giving countries, funders, procuring agencies and communities the assurance that it meets international standards for safety, efficacy and manufacturing. The vaccine is produced by the Beijing-based pharmaceutical company Sinovac.
“The world desperately needs multiple COVID-19 vaccines to address the huge access inequity across the globe,” said Dr Mariângela Simão, WHO Assistant-Director General for Access to Health Products. “We urge manufacturers to participate in the COVAX Facility, share their knowhow and data and contribute to bringing the pandemic under control.” WHO’s Emergency Use Listing (EUL) is a prerequisite for COVAX Facility vaccine supply and international procurement. It also allows countries to expedite their own regulatory approval to import and administer COVID-19 vaccines.
The EUL assesses the quality, safety and efficacy of COVID-19 vaccines, as well as risk management plans and programmatic suitability, such as cold chain requirements. The assessment is performed by the product evaluation group, composed by regulatory experts from around the world and a Technical Advisory Group (TAG), in charge of performing the risk-benefit assessment for an independent recommendation on whether a vaccine can be listed for emergency use and, if so, under which conditions. In the case of the Sinovac-CoronaVac vaccine, the WHO assessment included on-site inspections of the production facility. The Sinovac-CoronaVac product is an inactivated vaccine. Its easy storage requirements make it very manageable and particularly suitable for low-resource settings.
WHO’s Strategic Advisory Group of Experts on Immunization (SAGE) has also completed its review of the vaccine. On the basis of available evidence, WHO recommends the vaccine for use in adults 18 years and older, in a two-dose schedule with a spacing of two to four weeks. Vaccine efficacy results showed that the vaccine prevented symptomatic disease in 51% of those vaccinated and prevented severe COVID-19 and hospitalization in 100% of the studied population.
Few older adults (over 60 years) were enrolled in clinical trials, so efficacy could not be estimated in this age group. Nevertheless, WHO is not recommending an upper age limit for the vaccine because data collected during subsequent use in multiple countries and supportive immunogenicity data suggest the vaccine is likely to have a protective effect in older persons. There is no reason to believe that the vaccine has a different safety profile in older and younger populations. WHO recommends that countries using the vaccine in older age groups conduct safety and effectiveness monitoring to verify the expected impact and contribute to making the recommendation more robust for all countries.

By World Health Organisation
Category: Health, Industry
 No Comments
No Comments
WHO is bringing the world’s scientists and global health professionals together to accelerate the research and development process, and develop new norms and standards to contain the spread of the coronavirus pandemic and help care for those affected. The R&D Blueprint has been activated to accelerate diagnostics, vaccines and therapeutics for this novel coronavirus. The solidarity of all countries will be essential to ensure equitable access to COVID-19 health products.
Global research database WHO is gathering the latest international multilingual scientific findings and knowledge on COVID-19. The global literature cited in the WHO COVID-19 database is updated daily (Monday through Friday) from searches of bibliographic databases, hand searching, and the addition of other expert-referred scientific articles. This database represents a comprehensive multilingual source of current literature on the topic. While it may not be exhaustive, new research is added regularly.
The WHO evidence retrieval sub-group has begun collaboration with key partners to enrich the citations and build a more comprehensive database with inclusion of other content. The database is built by BIREME, the Specialized Center of PAHO/AMRO and part of the Regional Office’s Department of Evidence and Intelligence for Action in Health. For further information or questions, please contact the WHO Library via email.
Disclaimer: the designations employed and the presentation of the material in publications listed in this database does not imply the expression of any opinion whatsoever on the part of WHO concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted and dashed lines on maps represent approximate border lines for which there may not yet be full agreement.
The mention of specific companies or of certain manufacturers’ products in publications listed in the database does not imply that they are endorsed or recommended by WHO in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters.
By listing publications in this database and providing links to external sites does not mean that WHO endorses or recommends those publications or sites, or has verified the content contained within them. The database has been compiled without warranty of any kind, either expressed or implied. The responsibility for the interpretation and use of publications included in this database lies with the reader. In no event shall WHO be liable for damages arising from its use
November 21, 2019
November 21, 2020
November 21, 2021
Should you have any queries, please feel free to contact Premium Medical Services.
Contact Us